what happens to the hydrogen electrons at the end of the electron transport chain
Learning Outcomes
- Depict the respiratory concatenation (electron transport chain) and its function in cellular respiration
You have just read about two pathways in cellular respiration—glycolysis and the citric acrid bike—that generate ATP. However, nearly of the ATP generated during the aerobic catabolism of glucose is not generated direct from these pathways. Rather, information technology is derived from a procedure that begins with moving electrons through a serial of electron transporters that undergo redox reactions: the electron transport chain. This causes hydrogen ions to accumulate within the matrix infinite. Therefore, a concentration slope forms in which hydrogen ions diffuse out of the matrix space by passing through ATP synthase. The current of hydrogen ions powers the catalytic action of ATP synthase, which phosphorylates ADP, producing ATP.
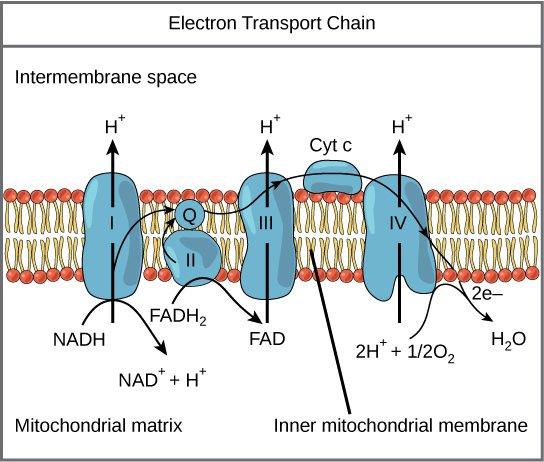
Figure 1. The electron ship chain is a series of electron transporters embedded in the inner mitochondrial membrane that shuttles electrons from NADH and FADHtwo to molecular oxygen. In the process, protons are pumped from the mitochondrial matrix to the intermembrane infinite, and oxygen is reduced to form water.
The electron transport chain (Effigy 1) is the last component of aerobic respiration and is the merely part of glucose metabolism that uses atmospheric oxygen. Oxygen continuously diffuses into plants; in animals, it enters the torso through the respiratory system. Electron transport is a serial of redox reactions that resemble a relay race or bucket brigade in that electrons are passed apace from one component to the side by side, to the endpoint of the chain where the electrons reduce molecular oxygen, producing h2o. There are four complexes equanimous of proteins, labeled I through IV in Effigy i, and the aggregation of these iv complexes, together with associated mobile, accessory electron carriers, is called the electron transport concatenation. The electron transport chain is present in multiple copies in the inner mitochondrial membrane of eukaryotes and the plasma membrane of prokaryotes. Annotation, nevertheless, that the electron transport chain of prokaryotes may non require oxygen as some live in anaerobic weather. The common feature of all electron transport bondage is the presence of a proton pump to create a proton gradient across a membrane.
Circuitous I
To showtime, ii electrons are carried to the kickoff complex aboard NADH. This circuitous, labeled I, is composed of flavin mononucleotide (FMN) and an iron-sulfur (Fe-S)-containing protein. FMN, which is derived from vitamin B2, also called riboflavin, is i of several prosthetic groups or co-factors in the electron transport chain. Aprosthetic group is a non-protein molecule required for the activity of a protein. Prosthetic groups are organic or inorganic, non-peptide molecules leap to a protein that facilitate its function; prosthetic groups include co-enzymes, which are the prosthetic groups of enzymes. The enzyme in complex I is NADH dehydrogenase and is a very large protein, containing 45 amino acid chains. Complex I can pump four hydrogen ions across the membrane from the matrix into the intermembrane space, and it is in this way that the hydrogen ion gradient is established and maintained betwixt the 2 compartments separated by the inner mitochondrial membrane.
Q and Complex II
Complex Two directly receives FADHtwo, which does not pass through complex I. The compound connecting the first and 2nd complexes to the third isubiquinone (Q). The Q molecule is lipid soluble and freely moves through the hydrophobic core of the membrane. In one case information technology is reduced, (QHtwo), ubiquinone delivers its electrons to the adjacent complex in the electron ship concatenation. Q receives the electrons derived from NADH from circuitous I and the electrons derived from FADH2 from complex II, including succinate dehydrogenase. This enzyme and FADH2 form a small circuitous that delivers electrons direct to the electron send chain, bypassing the starting time circuitous. Since these electrons bypass and thus exercise not energize the proton pump in the get-go complex, fewer ATP molecules are made from the FADH2 electrons. The number of ATP molecules ultimately obtained is directly proportional to the number of protons pumped across the inner mitochondrial membrane.
Complex III
The third circuitous is composed of cytochrome b, another Iron-S protein, Rieske center (2Fe-2S middle), and cytochrome c proteins; this complex is also called cytochrome oxidoreductase. Cytochrome proteins take a prosthetic group of heme. The heme molecule is similar to the heme in hemoglobin, but it carries electrons, not oxygen. Equally a result, the iron ion at its core is reduced and oxidized as it passes the electrons, fluctuating between different oxidation states: Fe+ + (reduced) and Fe+ + + (oxidized). The heme molecules in the cytochromes take slightly different characteristics due to the effects of the different proteins binding them, giving slightly different characteristics to each complex. Circuitous Iii pumps protons through the membrane and passes its electrons to cytochrome c for transport to the fourth complex of proteins and enzymes (cytochrome c is the acceptor of electrons from Q; however, whereas Q carries pairs of electrons, cytochrome c can accept only one at a fourth dimension).
Complex IV
The fourth complex is composed of cytochrome proteins c, a, and a3. This complex contains ii heme groups (one in each of the two cytochromes, a, and a3) and three copper ions (a pair of CuA and one CuB in cytochrome a3). The cytochromes concord an oxygen molecule very tightly between the fe and copper ions until the oxygen is completely reduced. The reduced oxygen and then picks up 2 hydrogen ions from the surrounding medium to make h2o (HiiO). The removal of the hydrogen ions from the system contributes to the ion gradient used in the process of chemiosmosis.
Chemiosmosis
In chemiosmosis, the costless energy from the series of redox reactions just described is used to pump hydrogen ions (protons) beyond the membrane. The uneven distribution of H+ ions across the membrane establishes both concentration and electrical gradients (thus, an electrochemical gradient), owing to the hydrogen ions' positive charge and their aggregation on one side of the membrane.
If the membrane were open up to diffusion past the hydrogen ions, the ions would tend to diffuse back beyond into the matrix, driven past their electrochemical gradient. Retrieve that many ions cannot lengthened through the nonpolar regions of phospholipid membranes without the aid of ion channels. Similarly, hydrogen ions in the matrix space can only pass through the inner mitochondrial membrane through an integral membrane poly peptide called ATP synthase (Effigy two). This complex poly peptide acts as a tiny generator, turned by the force of the hydrogen ions diffusing through it, down their electrochemical gradient. The turning of parts of this molecular machine facilitates the addition of a phosphate to ADP, forming ATP, using the potential energy of the hydrogen ion gradient.
Practice Question
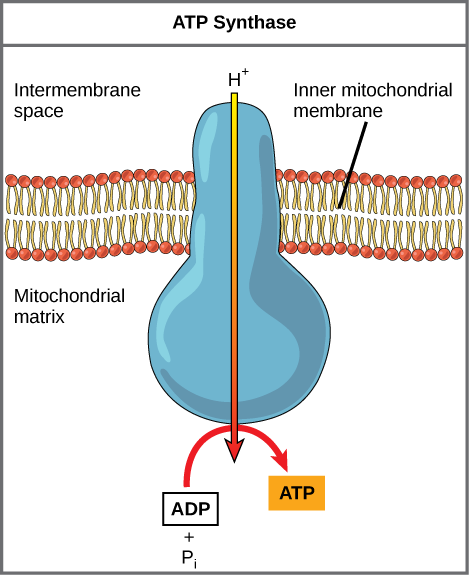
Figure 2. ATP synthase is a complex, molecular automobile that uses a proton (H+) gradient to form ATP from ADP and inorganic phosphate (Pi). (Credit: modification of work by Klaus Hoffmeier)
Dinitrophenol (DNP) is an uncoupler that makes the inner mitochondrial membrane leaky to protons. It was used until 1938 as a weight-loss drug. What effect would you look DNP to have on the alter in pH across the inner mitochondrial membrane? Why do you remember this might be an effective weight-loss drug?
Show Answer
After DNP poisoning, the electron send concatenation can no longer form a proton slope, and ATP synthase can no longer make ATP. DNP is an effective diet drug because it uncouples ATP synthesis; in other words, after taking information technology, a person obtains less energy out of the food he or she eats. Interestingly, one of the worst side effects of this drug is hyperthermia, or overheating of the body. Since ATP cannot be formed, the energy from electron ship is lost as heat.
Chemiosmosis (Figure 3) is used to generate 90 percentage of the ATP fabricated during aerobic glucose catabolism; it is also the method used in the light reactions of photosynthesis to harness the energy of sunlight in the process of photophosphorylation. Recall that the production of ATP using the process of chemiosmosis in mitochondria is called oxidative phosphorylation. The overall effect of these reactions is the product of ATP from the energy of the electrons removed from hydrogen atoms. These atoms were originally part of a glucose molecule. At the cease of the pathway, the electrons are used to reduce an oxygen molecule to oxygen ions. The extra electrons on the oxygen attract hydrogen ions (protons) from the surrounding medium, and water is formed.
Practice Question
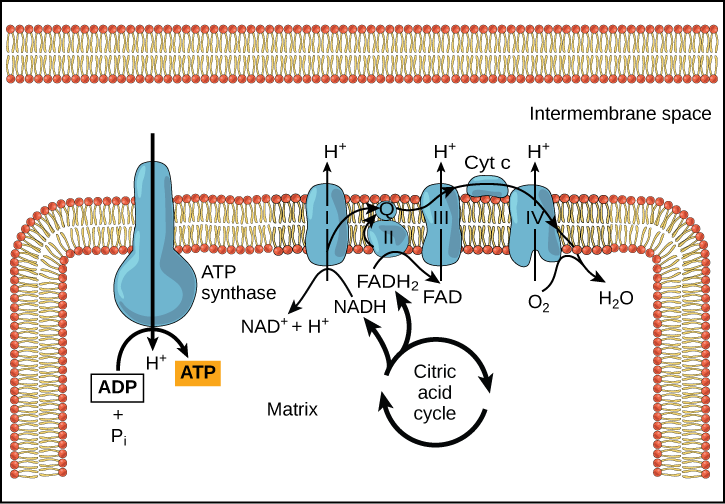
Figure three. In oxidative phosphorylation, the pH gradient formed past the electron ship chain is used by ATP synthase to grade ATP.
Cyanide inhibits cytochrome c oxidase, a component of the electron transport chain. If cyanide poisoning occurs, would you lot expect the pH of the intermembrane space to increase or decrease? What effect would cyanide have on ATP synthesis?
Evidence Answer
Afterward cyanide poisoning, the electron transport chain tin no longer pump electrons into the intermembrane space. The pH of the intermembrane infinite would increase, the pH gradient would decrease, and ATP synthesis would stop.
ATP Yield
The number of ATP molecules generated from the catabolism of glucose varies. For case, the number of hydrogen ions that the electron transport concatenation complexes can pump through the membrane varies between species. Another source of variance stems from the shuttle of electrons across the membranes of the mitochondria. (The NADH generated from glycolysis cannot easily enter mitochondria.) Thus, electrons are picked upwards on the within of mitochondria past either NAD+ or FAD+. Equally y'all have learned before, these FAD+ molecules can transport fewer ions; consequently, fewer ATP molecules are generated when FAD+ acts as a carrier. NAD+ is used every bit the electron transporter in the liver and FAD+ acts in the brain.
Another gene that affects the yield of ATP molecules generated from glucose is the fact that intermediate compounds in these pathways are used for other purposes. Glucose catabolism connects with the pathways that build or suspension down all other biochemical compounds in cells, and the result is somewhat messier than the ideal situations described thus far. For case, sugars other than glucose are fed into the glycolytic pathway for free energy extraction. Moreover, the five-carbon sugars that class nucleic acids are fabricated from intermediates in glycolysis. Sure nonessential amino acids can be made from intermediates of both glycolysis and the citric acid cycle. Lipids, such equally cholesterol and triglycerides, are also made from intermediates in these pathways, and both amino acids and triglycerides are broken downwardly for energy through these pathways. Overall, in living systems, these pathways of glucose catabolism extract about 34 percent of the energy independent in glucose.
In Summary: Electron Transport Concatenation
The electron send chain is the portion of aerobic respiration that uses free oxygen as the final electron acceptor of the electrons removed from the intermediate compounds in glucose catabolism. The electron transport chain is composed of four large, multiprotein complexes embedded in the inner mitochondrial membrane and ii modest diffusible electron carriers shuttling electrons between them. The electrons are passed through a series of redox reactions, with a pocket-size amount of free energy used at iii points to transport hydrogen ions across a membrane. This procedure contributes to the gradient used in chemiosmosis. The electrons passing through the electron transport chain gradually lose energy, High-free energy electrons donated to the chain by either NADH or FADH2 complete the concatenation, as low-energy electrons reduce oxygen molecules and grade water. The level of costless energy of the electrons drops from nearly 60 kcal/mol in NADH or 45 kcal/mol in FADH2 to about 0 kcal/mol in water. The end products of the electron transport concatenation are water and ATP. A number of intermediate compounds of the citric acid cycle can exist diverted into the anabolism of other biochemical molecules, such equally nonessential amino acids, sugars, and lipids. These same molecules can serve equally energy sources for the glucose pathways.
Try It
Contribute!
Did you have an idea for improving this content? We'd dearest your input.
Amend this pageLearn More
Source: https://courses.lumenlearning.com/wm-biology1/chapter/reading-electron-transport-chain/
0 Response to "what happens to the hydrogen electrons at the end of the electron transport chain"
Post a Comment